Images Genevieve Carbonatto. Text Genevieve Carbonatto
A young man presents to the Emergency Department with SOB (shortness of breath). He has been sent in by his flatmates because he is so unwell. They leave as soon as he presents to triage. He is an intravenous drug abuser, but says he hasn’t taken anything for 2 weeks. He is not very communicative. When asked he says he has been unwell for 3 weeks. He thinks he has had fevers. On examination he is very lethargic. Temperature 37.8, HR 100/min, BP 95/60, chest clear, breath sounds vesicular. On auscultation a loud murmur is heard throughout his chest radiating to the carotids.
Ok so : Fever + IVDU + heart murmur = infective endocarditis until proven otherwise.
You check for petechiae on his skin, his arms, his legs, his trunk. You check for splinter haemorrhages (nail beds), you feel for Osler’s nodes (tender subcuteous nodules in pulps of fingers) and look for Janeway lesions on his palms and soles (non tender maculae on his palms and soles). He has a couple of splinter haemorrhages and there are some petechiae on his lower limbs – just 3 or 4 you think. You know about Roth spots, but you don’t look. You order 3 sets of blood cultures, a full blood count, electrolytes and a CRP. A chest Xray is obtained.
You get your ultrasound machine and you perform an echo.
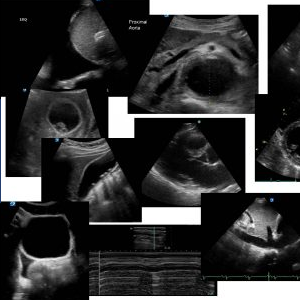
A good parasternal long axis, (PLAX) view is obtained.
On PLAX, several large friable structures are visualised on the aortic valve leaflets. They are moving erratically and independently of the valve motion. New vegetations are the same echogenicity as the myocardium. Vegetations are generally attached to the upstream side of valves, i.e. LV side of aortic valve and atrial side of mitral valve in the left side of the heart. In the presence of infected regurgitant jet(s), satellite vegetations may be found wherever the jet passes / impinges. A clinically insignificant amount of pericardial fluid is seen in all the following images.
Parasternal short axis at the level of the left ventricular outflow tract, (LVOT) showing the thickened anterior mitral leaflet (AMVL) with the vegetation moving erractically from the aortic leaflets into the LVOT. Look for vegetations on the anterior mitral leaflet, (AMVL) or perforations of the AMVL which may be satellite vegetations from aortic regurgitation (AR).
Parasternal short axis at the level of the aortic valve. The aortic valve is trileaflet and mildly thickened which are most likely the bases of vegetations on the valve leaflets. Look for perforations, pseudoaneurysms of the aortic root and aortic root abscesses in these cases. The first evidence of aortic root abscess will be thickening of the aortic root wall.
Apical 4-chamber view. No definite vegetations are seen on the mitral valve in this image but the valve leaflet looks slightly thickened. Always use the highest frequency probe available and zoom images when looking for vegetations on valves and / or for satellite vegetations. The tricuspid leaflets are not well displayed in this image. The LV and LA are dilated.
Apical 5-chamber view demonstrating the LVOT and aortic valve with several vegetations on both aortic leaflets. The length of the vegetations are well displayed on this image and the tricuspid valve leaflets are well seen.
Apical 5-chamber view with colour across the aortic valve. A significant aortic regurgitation, (AR) jet would be expected. Use the colour to pan across the valve to look for the largest regurgitant jet. Look for satellite vegetations in the path of the infected AR. This image appears to be on the edge of the regurgitation.
Apical 4-chamber view. There is no definite evidence of mobile vegetations on the tricuspid or mitral valve. No satellite vegetations are visualised on the interventricular septum. The RV is heavily trabeculated. The LV appears to be mildly dilated on all views. This, in relation to the patients body surface area, (BSA) is significant.
Subcostal 4-chamber view. There is no evidence of vegetations on the tricuspid or mitral valve on TTE. No satellite vegetations are visualised on the interventricular septum.
Although the aortic valve vegetations was clearly seen with a Point of Care echo, a formal transthoracic echo also identified a possible anterior mitral valve leaflet vegetation which was confirmed on transoesophageal echo.
Teaching point: Echocardiography is the best imaging modality for infective endocarditis. This applies mainly to transoesophageal echo (TOE) which has a 90 – 100% detection rate, however transthoracic echo (TTE) has been found to have a detection rate of 50% in finding a vegetation in patients with a clinical suspicion of infective endocarditis. It is definitely worth doing in the emergency department if the clinical suspicion is high. Remember that vegetations are not always mobile, moving erratically with the movement of the valve, they may be sessile and present simply as a non mobile mass on a valve.
Discussion:
In infective endocarditis the vegetation is a mixture of thrombus, bacteria and inflammatory cells. The infection on the valves or the endocardium essentially “liquifies” the valves causing perforations within the valves or the endocardium. Abscess formation and prosthetic valve dehiscence are the most common other pathological findings. More rarely, fistulas between cardiac cavities, valve aneurysms (saccular outpouching of valvular tissue), fistulas and pseudoaneurysms (perivalvular cavity communicating with the cardiovascular lumen) can occur.
The epidemiology of bacterial endocarditis has changed over the last 50 years worldwide. A systematic review (2) demonstrated that the number of staphylococcal infections, coagulase negative staphylococcas and staphylococcus aureus (SA) increased over the last 50 years (SA from 21% to 30%). Streptococcus viridans and culture negative infective endocarditis decreased over this period while enterococci increased over the last 10 years. Patient age for bacterial endocarditis and male predominance also has increased. Whilst the average age for infective endocarditis in the 1940’s was 35, now it is 55, with a high number of elderly patients affected. The causes for this changing epidemiology is thought to be due to the increase in intravenous drug abuse (especially in the United States), the increase in age of patients overall, the higher number of chronically ill patients, increased contacts with the health care system and increasing use of intracardiac and vascular devices.
A more local Australian study looking at infective endocarditis and rheumatic fever in northern Australia (2012), showed that the principle risk for infective endocarditis now, is not rheumatic heart disease and that the risk of infective endocarditis is not restricted to indigenous Australians with rheumatic heart disease. 42% of patients with rheumatic heart disease and associated infective endocarditis were non Indigenous in the study. The commonest risk factors for infective endocarditis were prosthetic material, IV drug use and previous infective endocarditis.
What does this mean for us as Emergency Physicians?
We need to think of infective endocarditis in the differential diagnosis of fever, worsening heart failure or constitutional symptoms not only in patients who are intravenous drug abusers or patients with rheumatic fever but also in older patients with underlying valve disease or prosthetic valves. Should we be doing point of care echo on all these patients groups?
References
- Heart, Lung, Circul. 2012 Jan;21(1):36-41. doi: 10.1016/j.hlc.2011.08.010. Epub 2011 Sep 15. Infective endocarditis and rheumatic heart disease in the north of Australia.
Baskerville CA1, Hanrahan BB, Burke AJ, Holwell AJ, Rémond MG, Maguire GP. - Infective Endocarditis Epidemiology Over Five Decades: A Systematic Review: Leandro Slipczuk, J. Nicolas Codolosa, Carlos D. Davila, Abel Romero-Corral, Jeong Yun, Gregg S. Pressman, Vincent M. Figueredo PLOS x Published: December 9, 2013http://dx.doi.org/10.1371/journal.pone.0082665
- Clinical infectious disease: Infective Endocarditis in Elderly Patients Vinod K. Dhawan
- Oral Surg Oral Med Oral Pathol 1992;73:30-4. : Felder RS, Nardone D, Palac R. Prevalence of predisposing factors for endocarditis among an elderly institutionalized population.
- Heart: 2004 Jun; 90(6): 614–617.Echocardiography in infective endocarditis A Evangelista and M T Gonzalez-Alujas