ECHO images and text Genevieve Carbonatto
The following patient presented to the Emergency Department after a syncopal event walking up a flight of stairs. He had become more short of breath (SOB) over the past week or so, finding it hard to walk more than 50m without beocoming SOB. On arrival his vital signs are : BP 85/60, HR 98/min , oxygen saturations 97% on room air, respiratory rate 23/min .
This is his ECG

His ECG shows sinus rythm, right axis deviation and right bundle branch block. There is no evidence of ventricular hypertrophy
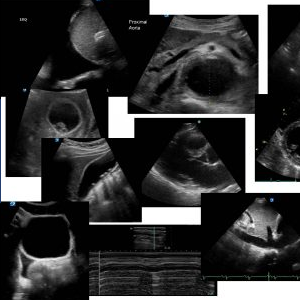
The following is his PLAX. Note that it is often very satisfying doing an echo on a patient with amyloidosis because of the hyperechoic nature of the myocardium which makes visualisation of the heart easier.
Note the thickened IVS and the “sparkling” myocardium. In this case the mitral valve and the aortic valves are minimally thickened. Compared to the aortic root both the left atrium and the right ventricle appear enlarged in this view. Note the LV is not dilated. LV contraction does appear to be reduced.
Even though colour and spectral Doppler are not standard for Emergency Echocardiography, it is interesting to note in this view that there is mild MR and in this clip, the thickening of the subvalvular apparatus is better appreciated.

Here the IVS measures 1.27 cm and the posterior LV wall 1.56 cm at the end of diastole (normal is up to 1.1cm)
This is a dedicated view of the PLAX right ventricular inflow. Note the TV is not significantly thickened.
RV / RA pressure measurements in this view show an RV / RA pressure gradient of 30.6, (31) mmHg. The IVC was fixed and dilated, so the true RV systolic pressure is closer to 46mmHg (this includes the estimated right atrial pressure of 15mmHg).

The PSAX view confirms increased right ventricular pressures. The IVS is flattened causing a “D shaped” appearance of the left ventricle.
This is his 4 chamber view.
Note the left ventricle does not appear to be dilated. The right ventricle however does . Both atria are enlarged. The LV has good radial contraction but poor longitudinal contraction. If you look at the anterior mitral valve leaflet it does not seem to rise much in systole, indicating that there is significant loss of the longitudinal contraction of the LV in systole. This is characteristic of cardiac amyloid. Note the left sided pleural effusion and the small right sided pericardial efusion.
His LVOT VTI is only 8.7 cm. A heart with an normal cardiac output should have an LVOT VTI between 18cm and 22 cm

This is a more dedicated RV 4 chamber view.
The RV pressure gradient here is higher than in the PLAX view because we are more in line with blood flow. The RV / RA pressure gradient is 36.6mmHg, (37mmHg) which when added to the right atrial pressure are added is closer to 52mmHg.

This is his IVC
His IVC is fixed and dilated measured 0.5cm – 3cm from the junction of the IVC with right atrium.
In summary this man exhibits many signs of amyloid cardiac disease. Hyperechoic myocardium, normal sized LV, enlarged atria, thickened myocardium, high RV pressures and a small pericardial effusion. His ongoing management included an MRI to confirm the diagnosis of amyloid disease.
How do we manage his hypotension?
Orthostatic hypotension is often due to autonomic neuropathy in patients with amyloidosis, but clearly an exacerbating factor can be poor cardiac output coupled with loop diuretics which may cause intravascular fluid depletion, so judicial iv fluid may help this situation. Orthostatic hypotension may respond to use of support stockings coupled with modest doses of fludrocortisone (grade C recommendation; level IX evidence.(8) In this patient’s case his hypotension was managed by careful fluid management and iv diuretics.
Discussion
Cardiac amyloidosis is not a rare presentation to our Emergency Department. Patients usually present with cardiac failure or arrythmias. This ECHO study is representative of patients presenting to our ED with amyloid heart disease. We will first descibe cardiac amyloid and then review the ECHO study.
Amyloidosis includes a group of diseases that have in common extracellular deposition of insoluble fibrillar proteins in organs and tissues (1) Cardiac amyloid disease refers to the infitration of the heart with these insoluble fibrils (2). This infiltration leads to a “stiff” heart, a restrictive type of cardiomyopathy. Cardiovascular amyloidosis can be primary, a part of systemic amyloidosis, or a result of chronic systemic diseases elsewhere in the body (1).
ECHO features (3,4,5,6)
- “Granular ” appearance or ‘sparkling” hyperechoic appearance of the myocardium. This is not specific to cardiac amyloidosis and is also dependent on the ultrasound machine used.
- Increased wall thickness of both vetricles. Wall thickening is usually concentric but may be assymetric
- Biatrial dilatation
- Interatrial wall thickening – in advanced cases
- Normal sized ventricles
- Ventricular dilatation in advanced cardiac amyloidosis
- Pericardial effusion usually in advanced cases
- +/- Valve thickening and thickening of papillary muscles
- Evidence of diastolic dysfunction
- LV systolic dysfunction in advanced stages
Symptoms
- Excertional dyspnea, weakness, fatigue, chest discomfort
- Congestive heart failure
- Peripheral oedema, congestive hepatomegaly, increased JVP, ascites in advanced disease (6)
- Pleural effusion secondary to congestive heart failure or due to amyloid infiltration of the pleura (6)
- Arrythmias – most commonly AF in 10 -15% of patients (6)
- Thromboembolism especially if AF is present
- Hypotension due to low cardiac output, autonomic neuropathy or impaired vascular tone due to amyloid infiltration
ECG:
Despite the ventricular wall thickness the ECG may show normal or low voltages consistent with an infiltrative process rather than myocyte hypertrophy (4)
Treatment and management (2,6,7):
- Treat failure with salt restriction, loop diuretics, aldosterone antoagonists such as spironolactone. However, their use may lead to under-filling of small and stiff LV with further reduction of already compromised cardiac output and consequently to hypotension, vertigo, syncope as well as prerenal worsening of renal function (7)
- ACE inhibitors and angiotensin receptor blockers need to be used with caution as small doses may lead to severe hypotension (7)(grade C recommendation; level IV evidence).
- B blockers not recommended as they may exacerbate hypotension.
- Digoxin and Calcium channel blockers have been found to bind to amyloid fibrils and may account for the increased susceptibility to digoxin toxicity and to haemodynamic deterioration with calcium channel blockers (7) (grade C recommendation; level IV evidence).
- Amiodarone is well tolerated when used for rate control therapy in patients with AF (6)
- Anticoagulation imperative for patients with AF as atrial thrombi are common. (6,7)
- Permanent pacemaker for patients with AV block. (6)
Teaching point: Cardiac amyloidosis can cause a unique form of restrictive cardiomyopathy. It is worth avoiding B blockers, ACE inhibitors, Calcium channel bloscers and Digoxin in the Emergency Department. Amiodarone has been found to be effective for rate control of AF and BiPAP or CPAP + loop diuretics for the treatment of heart failure. Careful fluid management is the cornestone of treatment of hypotension.
References
- Tex heart Inst J 2005; 32(2): 178–184. Amyloid Heart Disease New Frontiers and Insights in Pathophysiology, Diagnosis, and Management ;Walid Hassan, MD, FACC, Hani Al-Sergani, MD, FACC, Walid Mourad, MD, FCAP, and Rashed Tabbaa, MD, FACC
- Up to date: Treatment of amyloid cardiomyopathy
- Wiki ECHO: cardiac amyloidosis
- Journal of Cardiology, vol 54 ,issue 1, August 2009, Pages 162–166 Case report Echocardiographic features of cardiac amyloidosis presenting as endomyocardial disease in a 54-year-old male Michael E. Fealeya, William D. Edwards, MDb,Francis K. Buadi, MDc, Imran S. Syed, MDd, Martha Grogan, MDe
- Echocardiography: 1991 Mar;8(2):253-9. Two-dimensional echocardiography in myocardial amyloidosis. Picano E1, Pinamonti B, Ferdeghini EM, Landini L, Slavich G, Orlandini A, Marini C, Lattanzi F, Camerini F.
- Cor and Vasa, vol 55, issue 1; February 2013, Pages e60–e75 Review Article Cardiac amyloidosis: A comprehensive review Michal Fikrlea, Tomáš Palečeka, b, , , Petr Kuchynkaa, Eduard Němečeka, Lenka Bauerovác, Jan Straubd, Romana Ryšaváe
- Journal of the American College of Cardiology, 50 (2007), pp. 2101–2110 Evaluation and management of the cardiac amyloidosis. J.B. Selvanayagam, P.N. Hawkins, B. Paul, et al.
- British journal haematology:Guidelines on the diagnosis and management of AL amyloidosis 20 May 2004; Dr Jenny Bird, Avon Haematology Unit, Bristol Haematology and Oncology Centre, Horfield Road, Bristol BS2 3ED, UK.